Preparation of Aspirin from Salicylic acid
Aspirin
Acetyl Salicylic acid also known as aspirin , is one of the most widely used medication to reduce fever and also used as pain killer . It is an acetyl derivative of Salicylic acid . It is white in colour , crystalline in shape, weakly acidic substance which melts at 135 Celsius .Theory
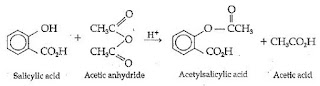
Aspirin is synthesised through the reaction of Salicylic acid with acetic anhydride which turns salicylic acid's hydroxyl group into an acetyl group .
A white , milky structure was obtained when Salicylic acid , acetic anhydride and sulphuric acid ( a catalyst ) were mixed .
the mechanism of the reaction is
In the first part of the experiment , Salicylic acid was heated so that it would melt and react with acetic anhydride .
Sulphuric acid is added as a catalyst . it is strong acid so there are many available protons. the synthesis is acid catalysed.
First there is a reversible reaction in which the the oxygen of carbonyl of acetic anhydride is protonated . the oxygen of phenol in salicylic acid acts as neucleophile and attacks the carbon of the carbonyl within acetic anhydride . this forms a tetrahedral intermediate . a series of proton transfers occurs . the electrons on the oxygen of the hydroxyl group on the tetrahedral intermediate ultimately comedown and reform a carbonyl group , this simultaneously includes an acetic acid to leave . after one additional proton transfer the final aspirin is formed.
Synthesis of aspirin
Uses Of Aspirin
Below are some of the therapeutic uses of aspirin :
- Mild to moderate pain
- Rheumatic fever
- Rheumatic arthritis
- In treatment of Pericarditis
- For prevention of stroke
Side Effects Of Aspirin
The most common side effects of aspirin are :
- Irritation of stomach or gut
- Indigestion
- Nausea
- Vomiting
How Does Aspirin Works ?
Aspirin ( acetylsalicylic aicd or ASP ) is a prostaglandin inhibitors that exhibits anti-inflammatory action . This anti-inflammatory action makes aspirin so effective in relieving pains and aches such as headache , toothache, backache and join paint and in reducing fever.

Comments
Post a Comment